Volkhard Lindner, MD, PhD
Faculty Scientist III
Center for Molecular Medicine
Lindner Lab
Deciphering the functions of CTHRC1 in the regulation of metabolism and tissue repair.
Collagen Triple Helix Repeat Containing-1 (CTHRC1) Functions
The focus of our laboratory is on understanding the function of the gene Collagen Triple Helix Repeat Containing-1 (Cthrc1), which was discovered in our laboratory as a gene expressed in tissues undergoing remodeling.

Figure 1. Cthrc1 is highly induced in the outer layer (adventitia) of arteries in response to injury. This layer undergoes a significant scarring process with abundant deposition of collagen, which leads to narrowing of the artery lumen.

Figure 2. CTHRC1 is not present in the normal heart (A), however, within 3 days following myocardial infarction CTHRC1 (in brown in B or green in C) is abundantly expressed in activated fibroblasts. Most of these myofibroblasts express both CTHRC1 and alpha-smooth muscle actin (in red in D).

Figure 3. CTHRC1 is expressed in bone by osteoblasts and osteocytes, not osteoclasts . (A) CTHRC1 is expressed in osteoblasts (arrowheads) and osteocytes of bone, whereas multinucleated osteoclasts (arrow) do not express CTHRC1. (B) Expression of the osteoclast marker TRAP (red) does not overlap with CTHRC1 (green) localization. (C-E) CTHRC1 is localized in cytoplasmic processes of osteocytes residing in the canaliculi. (F) Prominent accumulation of CTHRC1 is frequently seen around venules (arrows) suggesting potential release into circulation.
With genetic gain-of function and loss-of-function mouse models for Cthrc1 we seek to determine the functions of this molecule. Recent discoveries by our laboratory revealed that Cthrc1 functions as a predominantly bone-derived hormone (Figure 3) with substantial circulating levels detectable in some human subjects (Figure 6).
Evidence from genetic mouse models indicates that Cthrc1 plays a role in regulating body composition and voluntary physical activity. Increased body fat observed in Cthrc1 null mice is the result of inhibition of adipocyte differentiation by Cthrc1.


Figure 4. Cthrc1 null mice reveal increased visceral fat (in pink) and subcutaneous fat (in gray) as determined by microCT.

Figure 5. Inflammatory arthritis is exacerbated in Cthrc1 null mice. (A) In an inflammatory arthritis model CTHRC1 (brown) is expressed by the activated synoviocyte and in wildtype (WT) mice inflammation completely resolves without permanent joint damage (C). In Cthrc1 knockout mice no CTHRC1 is present in the inflamed joint (B) and massive inflammatory infiltrates persist with permanent damage to cartilage and bone (D).
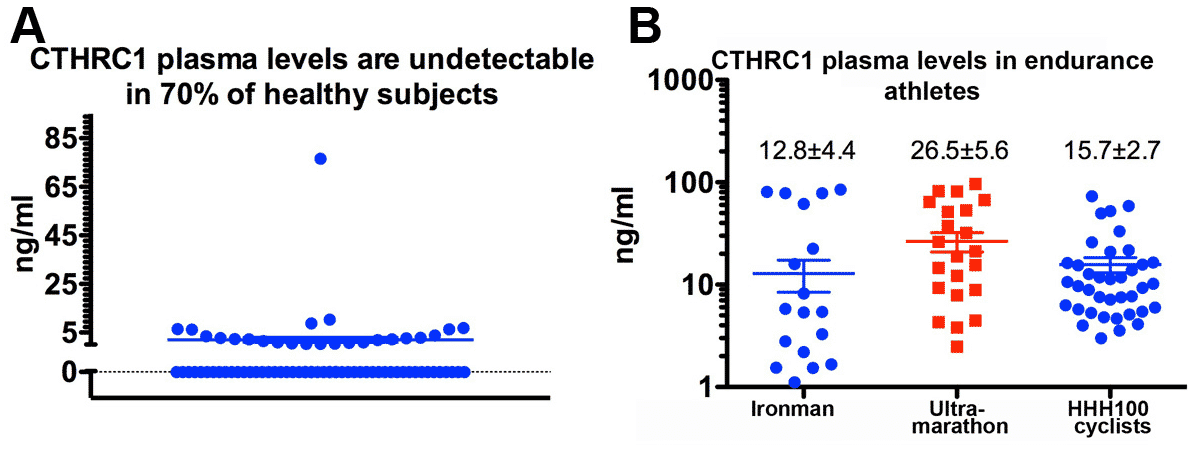
Figure 6. The highest average plasma levels of CTHRC1 were observed in champion endurance athletes. Ironman: World Championship 2013; Ultramarathon: Trans-Europe Foot Race; HHH: Hotter’n Hell Hundred (100 mile cycling race).
Our laboratory is interested in determining the role of CTHRC1 induced during tissue repair such as after myocardial infarction. In addition, we seek to understand the significance of circulating CTHRC1 in athletic performance and inflammation.